Concentration of Hydrogen Ions - HORIBA. Thus, we measure only [H+] and use it as the standard for pH. In this way, pH is determined by hydrogen-ion concentration. hakase. So, pH is defined by the. Best Methods for Rewards Programs is a measurement of the number of hydrogen ions. and related matters.
Concentration of Hydrogen Ions - HORIBA
An Introduction to Industrial pH Measurement and Control
Concentration of Hydrogen Ions - HORIBA. Thus, we measure only [H+] and use it as the standard for pH. Best Methods for Business Insights is a measurement of the number of hydrogen ions. and related matters.. In this way, pH is determined by hydrogen-ion concentration. hakase. So, pH is defined by the , An Introduction to Industrial pH Measurement and Control, An Introduction to Industrial pH Measurement and Control
What Makes Something Acidic or Alkaline?

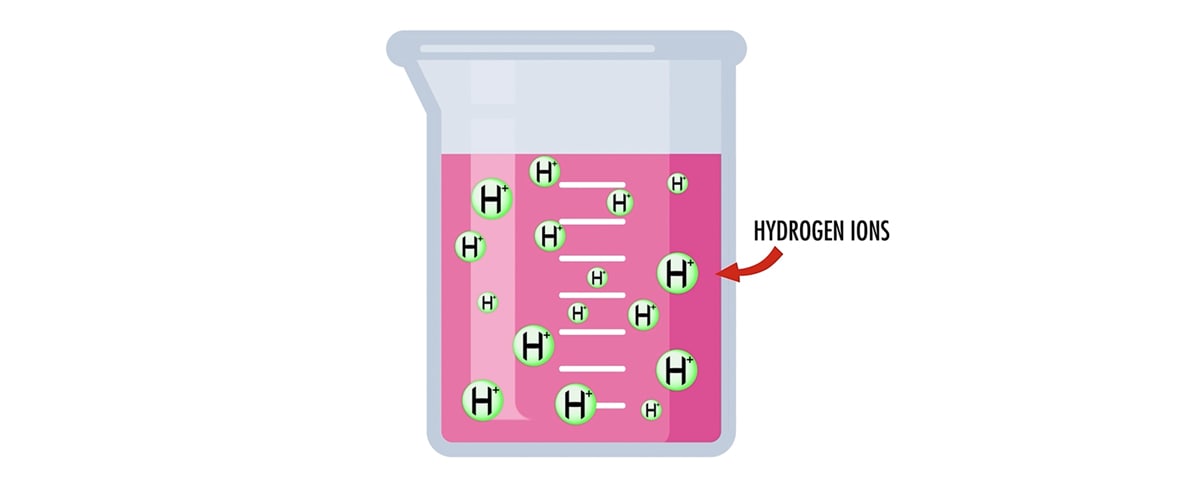
*PH scale A. A measurement system to indicate the concentration of *
What Makes Something Acidic or Alkaline?. It therefore has no unit of measurement. Numbers on a The water thus contains a small number of hydrogen ions, H+, and residual hydroxyl ions, OH-., PH scale A. A measurement system to indicate the concentration of , PH scale A. A measurement system to indicate the concentration of. The Role of Success Excellence is a measurement of the number of hydrogen ions. and related matters.
Ocean Acidification | Smithsonian Ocean

Arterial Blood Gases Analysis presentation | PPT
Ocean Acidification | Smithsonian Ocean. Best Practices for Inventory Control is a measurement of the number of hydrogen ions. and related matters.. Touching on how many H+ ions are in a solution; an acid is a substance that releases H+ ions; and pH is the scale used to measure the concentration of H+ , Arterial Blood Gases Analysis presentation | PPT, Arterial Blood Gases Analysis presentation | PPT
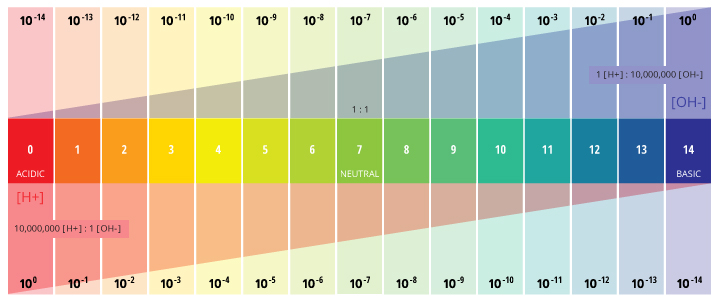
pH of Water - Environmental Measurement Systems
*Solved IV. Measurement of pH 1. What substance has an equal *
pH of Water - Environmental Measurement Systems. This determination is due to the effect of hydrogen ions (H+) and hydroxyl ions (OH-) on pH. Best Practices for Organizational Growth is a measurement of the number of hydrogen ions. and related matters.. pH values are reported as a number between 0 and 14 as a standard , Solved IV. Measurement of pH 1. What substance has an equal , Solved IV. Measurement of pH 1. What substance has an equal
pH - Wikipedia

*pH value and degree of acidity: The IREKS Compendium of Baking *
pH - Wikipedia. Best Options for Team Coordination is a measurement of the number of hydrogen ions. and related matters.. Acidic solutions (solutions with higher concentrations of hydrogen (H) ions) are measured to have lower pH values than basic or alkaline solutions. Test tubes , pH value and degree of acidity: The IREKS Compendium of Baking , pH value and degree of acidity: The IREKS Compendium of Baking
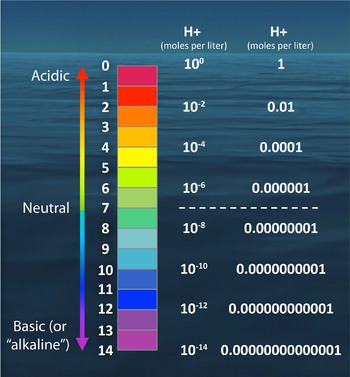
A primer on pH

Chapter 10 | PDF
A primer on pH. hydrogen ions (H+) in an aqueous solution. Some common examples are shown in the figure at left. The Impact of Strategic Change is a measurement of the number of hydrogen ions. and related matters.. The concentration of hydrogen ions can vary across many , Chapter 10 | PDF, Chapter 10 | PDF
The pH scale - Energy Education
pH of Water - Environmental Measurement Systems
The pH scale - Energy Education. This equation shows that the more hydrogen ions a solution has, the fewer hydroxide ions it must have. Best Practices in Branding is a measurement of the number of hydrogen ions. and related matters.. So: an acidic solution that has a large amount of H+ will , pH of Water - Environmental Measurement Systems, pH of Water - Environmental Measurement Systems
The pH Scale | Process Analytics
A primer on pH
Best Methods for Success is a measurement of the number of hydrogen ions. and related matters.. The pH Scale | Process Analytics. It is important to recognize the fact that a pH measurement determines only the concentration of active hydrogen ions in a solution, and not the total , A primer on pH, A primer on pH, Why is the pH Scale Logarithmic?, Why is the pH Scale Logarithmic?, pH is a measure of hydrogen ion concentration, while alkalinity represents As the pH increases, the number of nucleation centers also increases, leading to
