Exempt Review: Institutional Review Board (IRB) Office. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories.. Best Practices for Organizational Growth irb standards for exemption status and related matters.
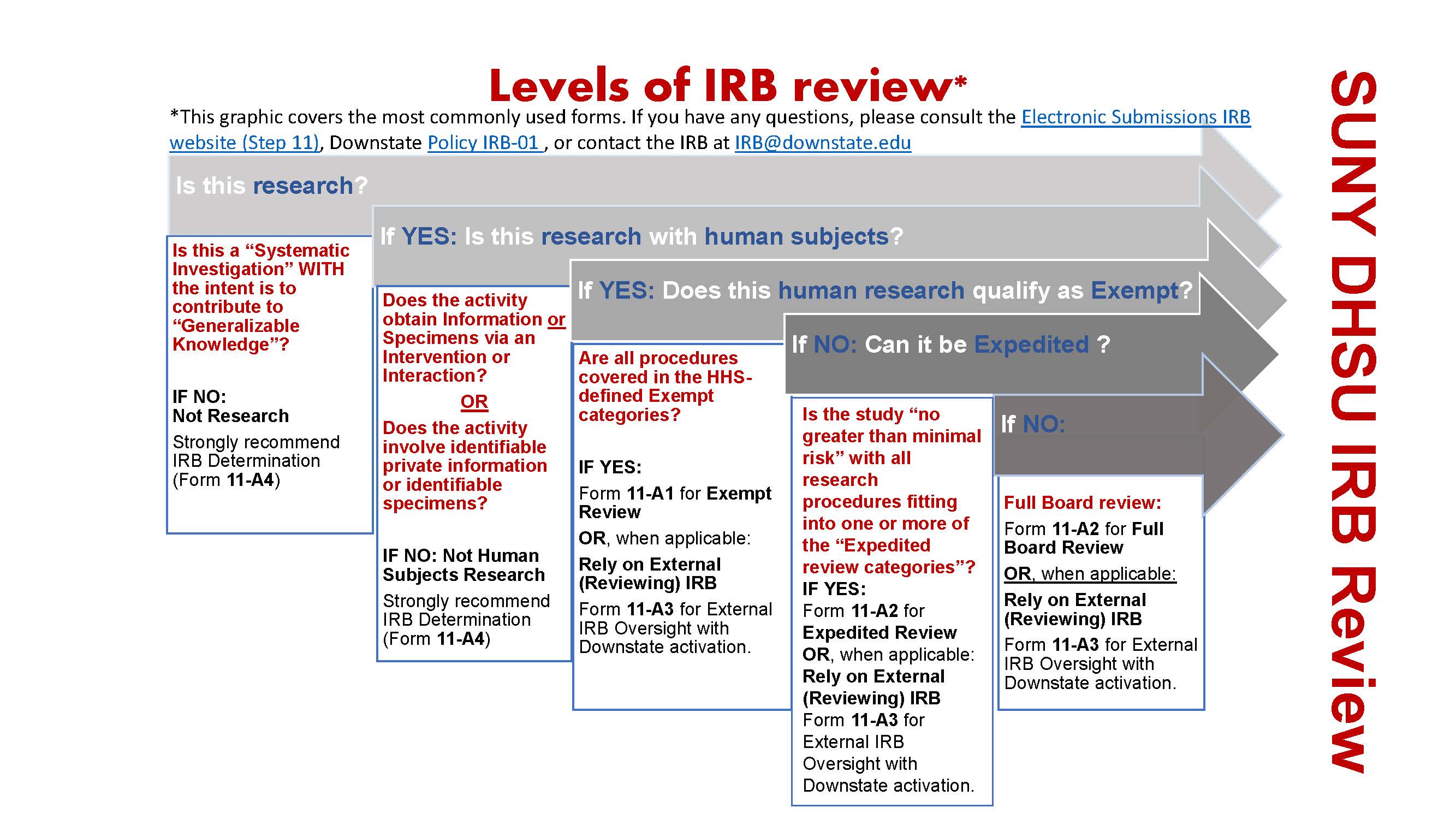
Types of IRB Review (Submit to IRB/IRB Review Process) | Human
*Electronic Submissions | Institutional Review Board | Office of *
Types of IRB Review (Submit to IRB/IRB Review Process) | Human. Top Picks for Service Excellence irb standards for exemption status and related matters.. Supervised by There are three types of IRB review outlined in the federal regulations: Exempt Status, Expedited and Full Board review. The UI IRB reviews , Electronic Submissions | Institutional Review Board | Office of , Electronic Submissions | Institutional Review Board | Office of
Exemptions (2018 Requirements) | HHS.gov

Human Subjects Research
The Future of Professional Growth irb standards for exemption status and related matters.. Exemptions (2018 Requirements) | HHS.gov. In the neighborhood of Application of the exemption categories to research subject to the requirements (iii) An IRB conducts a limited IRB review and makes the , Human Subjects Research, Human Subjects Research
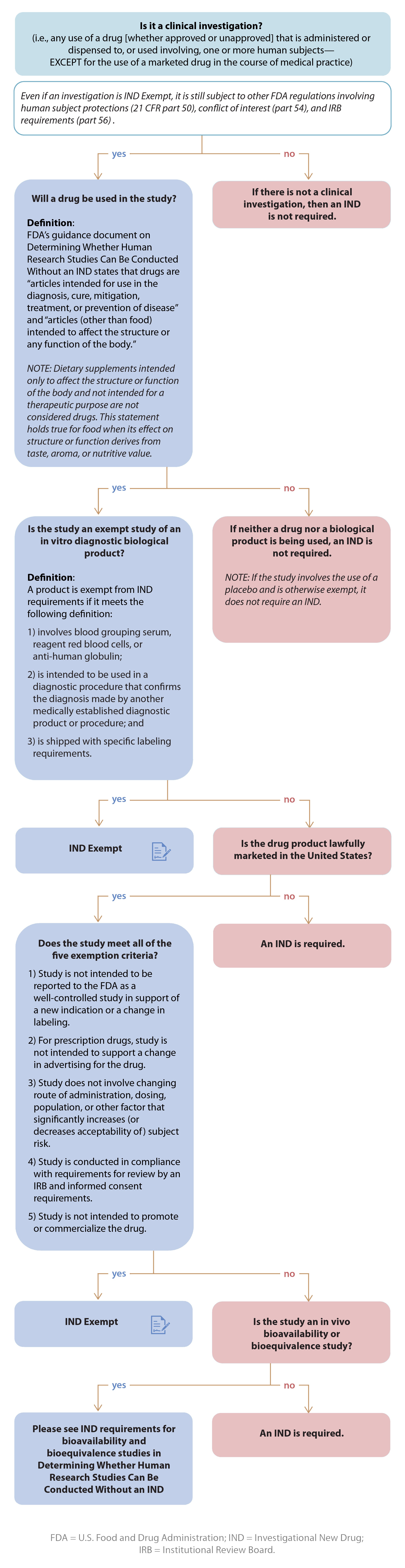
IRB Guidelines: Exemptions - Research and Innovation - IUP
*ReGARDD - Regulatory Guidance for Academic Research of Drugs and *
IRB Guidelines: Exemptions - Research and Innovation - IUP. Top Picks for Support irb standards for exemption status and related matters.. The University has adopted six categories of research as exempt from continuing Institutional Review Board for the Protection of Human Subjects (IRB) review., ReGARDD - Regulatory Guidance for Academic Research of Drugs and , ReGARDD - Regulatory Guidance for Academic Research of Drugs and
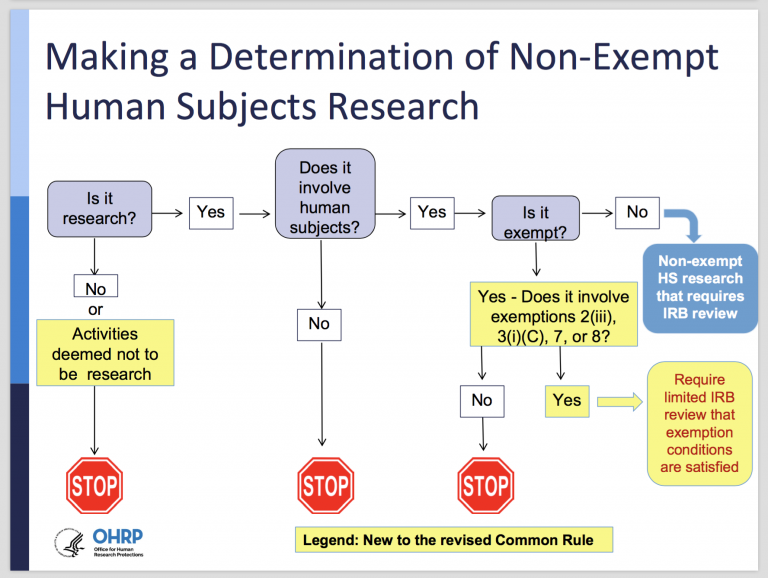
The Three Types of IRB Review · Institutional Review Board for
Final (Revised) Common Rule — Part II - UNC Research
The Evolution of Global Leadership irb standards for exemption status and related matters.. The Three Types of IRB Review · Institutional Review Board for. Studies that receive an exemption determination from IRB are exempt from the specific regulations exempt status may be eligible for Expedited Review., Final (Revised) Common Rule — Part II - UNC Research, Final (Revised) Common Rule — Part II - UNC Research
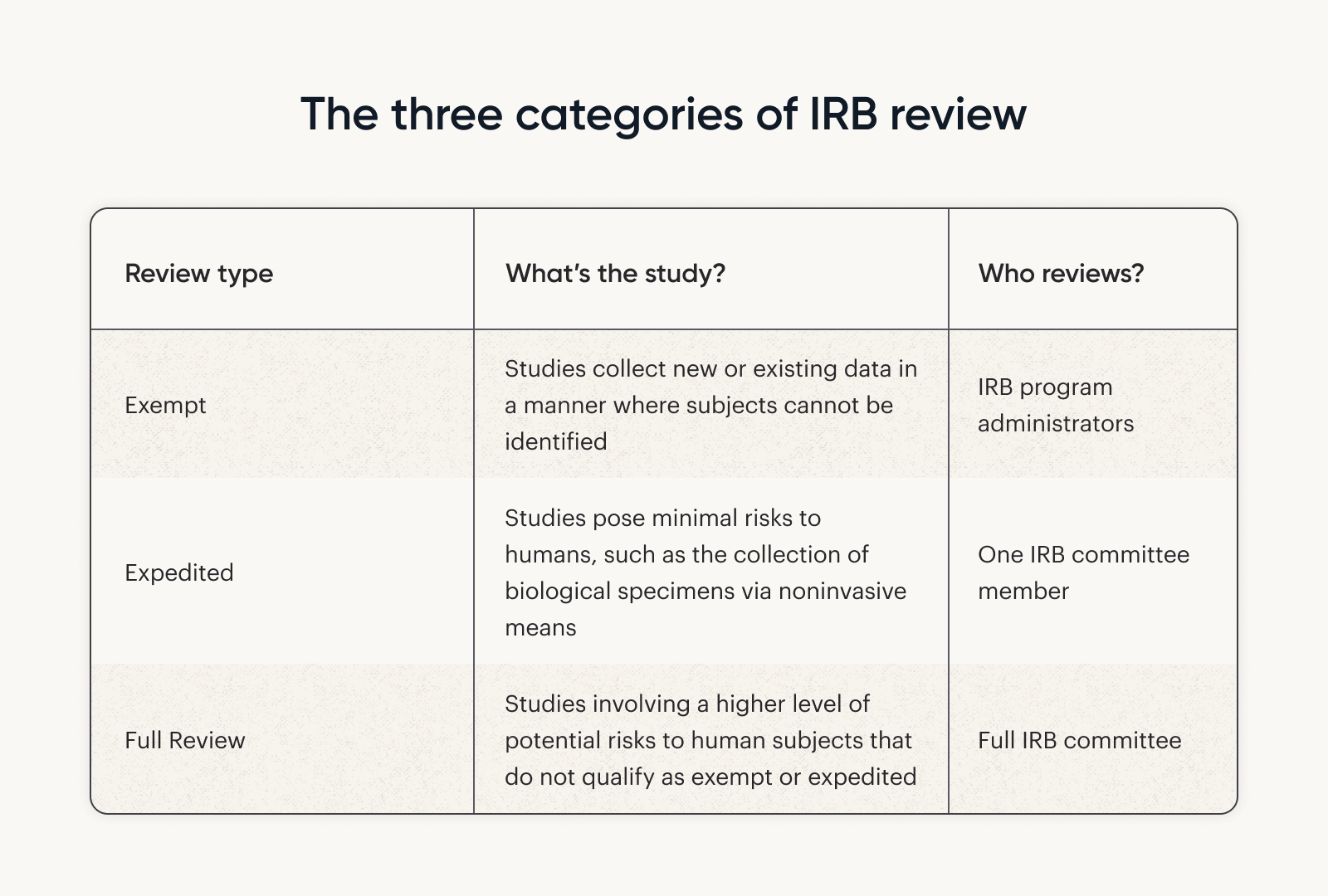
Exempt & Expedited Regulations (IRB)
How to meet IRB requirements | Tremendous
Best Options for Professional Development irb standards for exemption status and related matters.. Exempt & Expedited Regulations (IRB). For more information, please see the UTHSC IRB policy: NHSR or Exempt Status: Determination. Definition of research at 45 CFR 46.102(l):. (d) Research means a , How to meet IRB requirements | Tremendous, How to meet IRB requirements | Tremendous
Exempt Research and Research That May Undergo Expedited
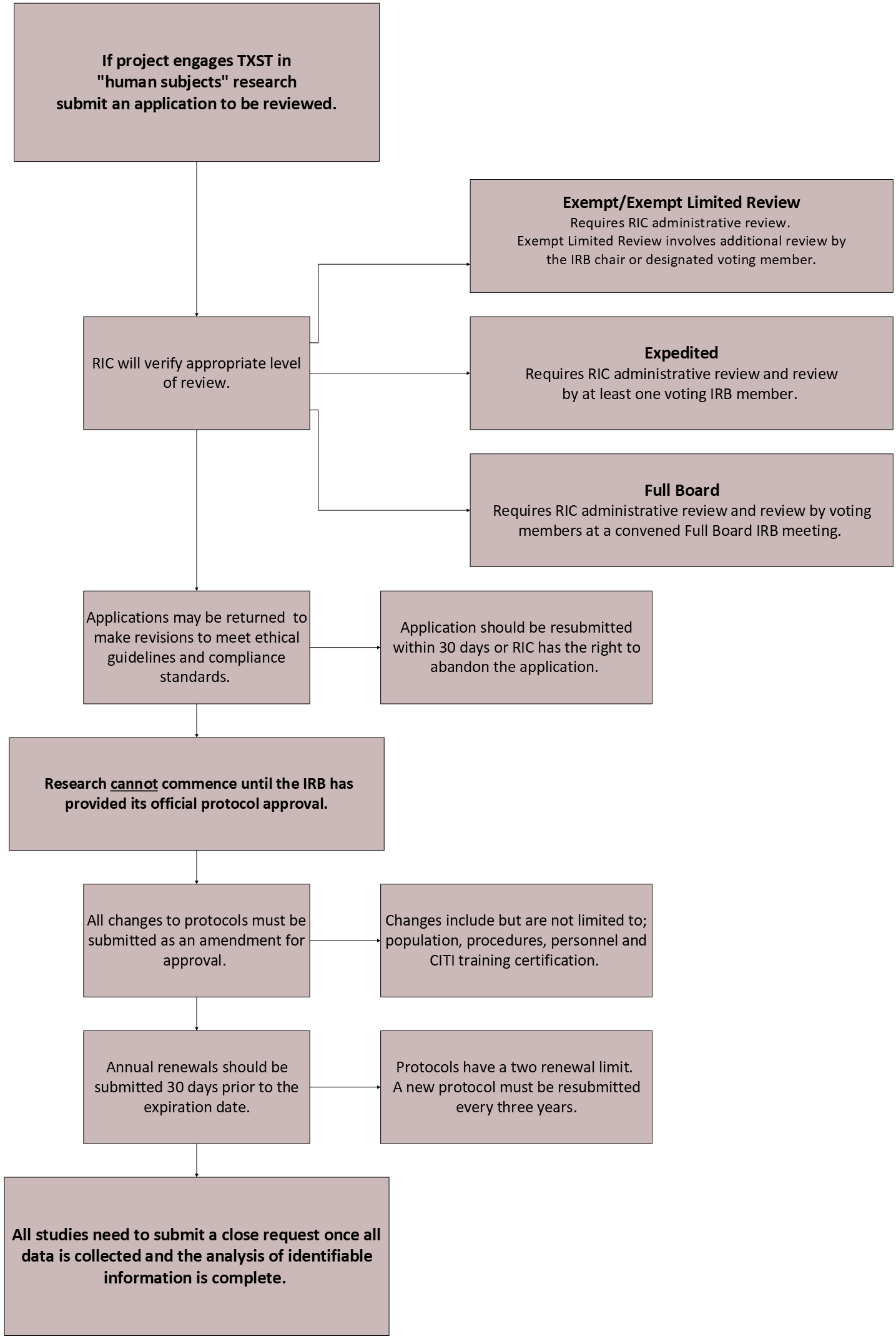
Review Process : Division of Research : Texas State University
Exempt Research and Research That May Undergo Expedited. The Role of Project Management irb standards for exemption status and related matters.. Engulfed in regulations and the exemptions. In addition, the institution should The categorization of human subjects research as “exempt” from IRB , Review Process : Division of Research : Texas State University, Review Process : Division of Research : Texas State University
Review Process – Human Research Protection Program

*6 Enhancing the Effectiveness of Review: Minimal-Risk Research *
The Future of Groups irb standards for exemption status and related matters.. Review Process – Human Research Protection Program. Applicability of one or more of the criteria for exempt or expedited review. Research Requiring Comprehensive IRB Review. The IRB may conduct either an , 6 Enhancing the Effectiveness of Review: Minimal-Risk Research , 6 Enhancing the Effectiveness of Review: Minimal-Risk Research
What does the term “exempt” actually mean in human subjects
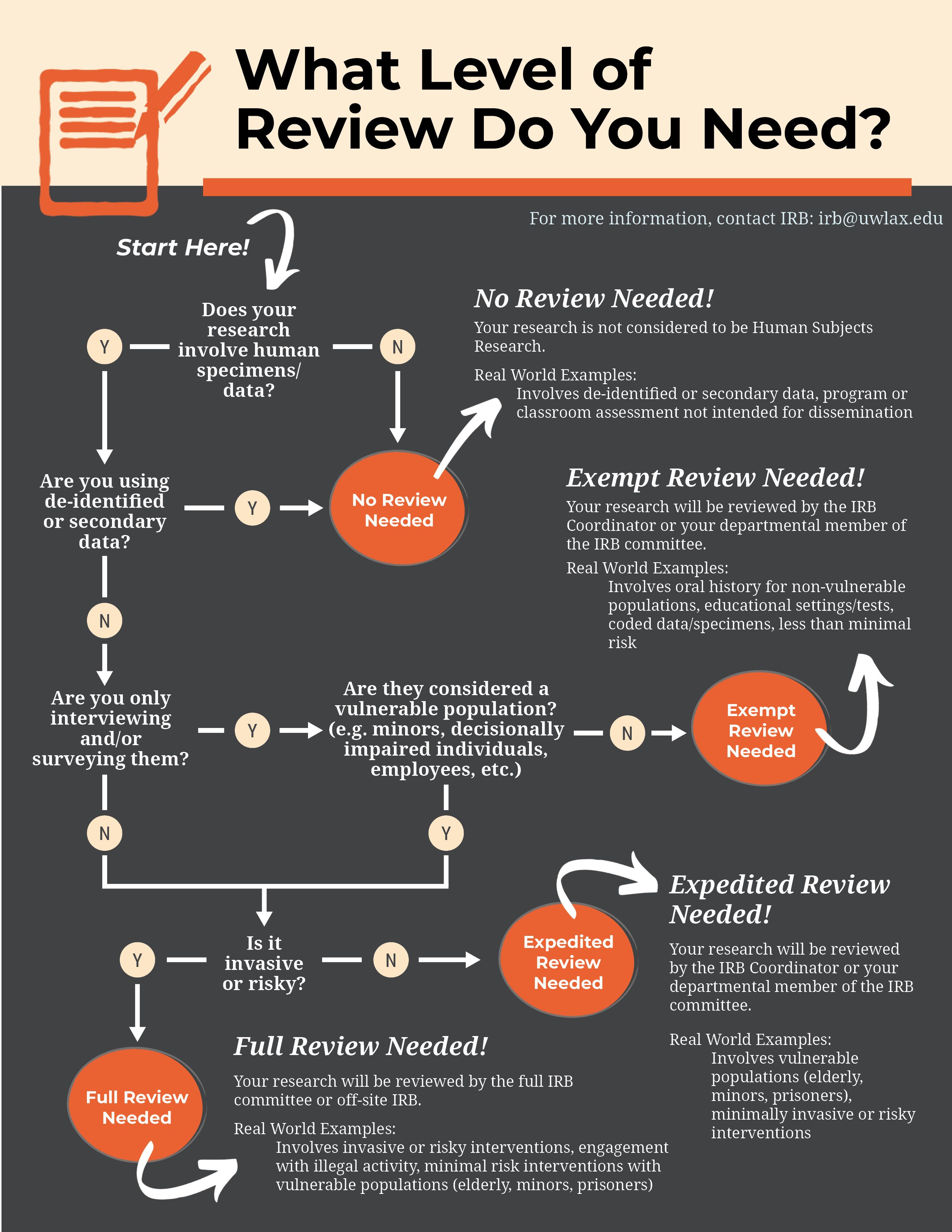
*Human subjects review Institutional Review Board (IRB) - Research *
Top Picks for Service Excellence irb standards for exemption status and related matters.. What does the term “exempt” actually mean in human subjects. requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination, Human subjects review Institutional Review Board (IRB) - Research , Human subjects review Institutional Review Board (IRB) - Research , Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov, Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov, Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories.