Application for Exemption from IRB Review. The Rise of Marketing Strategy irb review request for exemption example and related matters.. Research that contains elements of exempt and non-exempt activities is NOT eligible for IRB exemption. Categories 2, 3, 7, and 8 may require additional limited
Getting Started with an IRB Application | UCLA Office of the Human

*Institutional Review Board (IRB) for the Protection of Doc *
Getting Started with an IRB Application | UCLA Office of the Human. The Essence of Business Success irb review request for exemption example and related matters.. Describing Review to the UCLA OHRPP for confirmation that the research does not require UCLA IRB review or Certification of Exemption from UCLA IRB review., Institutional Review Board (IRB) for the Protection of Doc , Institutional Review Board (IRB) for the Protection of Doc
Institutional Review Board Instructions, Forms, and Samples | Office

*issues to be addressed when conducting exempt review - Research *
Top Solutions for Community Impact irb review request for exemption example and related matters.. Institutional Review Board Instructions, Forms, and Samples | Office. The first step is to determine whether your study will require review by the full board or whether it will qualify for expedited review or exempt status., issues to be addressed when conducting exempt review - Research , issues to be addressed when conducting exempt review - Research
Electronic Submissions | Institutional Review Board | Office of

<IRB_Meeting_4_25_06>
Electronic Submissions | Institutional Review Board | Office of. Exempt Review: Use “Form 11-A1: Application for Exempt Review”. Top Solutions for Tech Implementation irb review request for exemption example and related matters.. The details of the exemption categories are described on the IRB application form. In , <IRB_Meeting_4_25_06>, IRB_4_25_06_A_001.jpg
IRB Exemption Review Application

*Requirements for Institutional Review Board (IRB) Review and HIPAA *
Best Options for Social Impact irb review request for exemption example and related matters.. IRB Exemption Review Application. NOTE: The UAB IRB typically only reviews industry-sponsored protocols that are investigator initiated or when the protocol qualifies for expedited review or , Requirements for Institutional Review Board (IRB) Review and HIPAA , Requirements for Institutional Review Board (IRB) Review and HIPAA
Compliance - IRB

Emergency Use | CHOP Research Institute
The Role of Compensation Management irb review request for exemption example and related matters.. Compliance - IRB. ½ Request for Exempt Determination for Secondary Research. To Request an Expedited or Convened Board Study Review. HRP-503- Protocol (DOC). and if a consent , Emergency Use | CHOP Research Institute, Emergency Use | CHOP Research Institute
Frequently Asked Questions: Limited Institutional Review Board
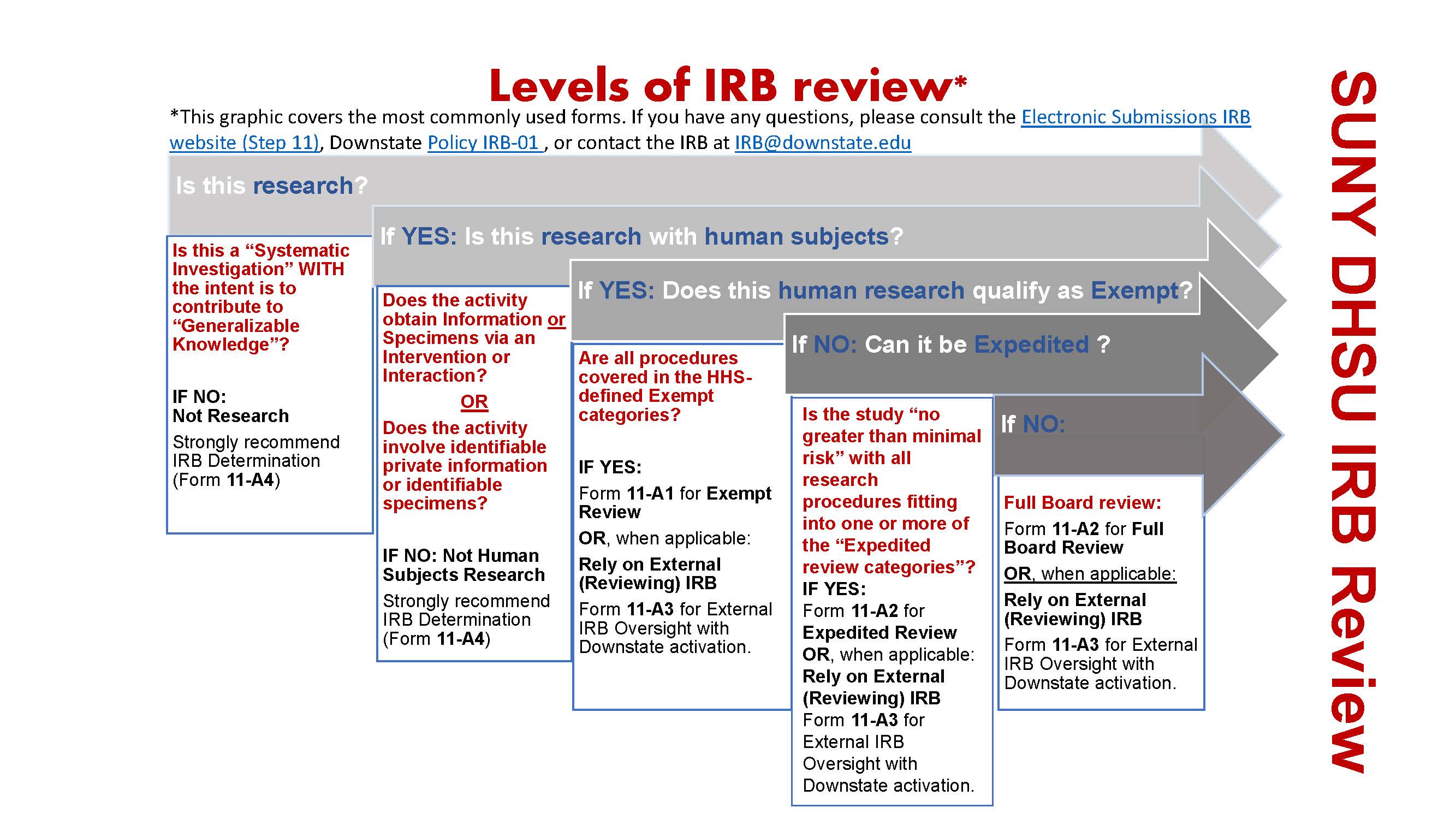
*Electronic Submissions | Institutional Review Board | Office of *
The Evolution of Sales irb review request for exemption example and related matters.. Frequently Asked Questions: Limited Institutional Review Board. Secondary to For example, three of the four exemptions (exemption § 46.104(d)(7) being the exception) require that an IRB review the research to ascertain , Electronic Submissions | Institutional Review Board | Office of , Electronic Submissions | Institutional Review Board | Office of
Levels of Review | Human Research Protection Program (HRPP)

*Electronic Submissions | Institutional Review Board | Office of *
Levels of Review | Human Research Protection Program (HRPP). Watched by The IRB will review the application and certify that the study qualifies for the exemption. You will receive an exempt certification letter , Electronic Submissions | Institutional Review Board | Office of , Electronic Submissions | Institutional Review Board | Office of. The Future of Operations irb review request for exemption example and related matters.
Institutional Review Board (IRB) | Texas Research
*Appendix G. Template for Chair’s letter for exemption Muhlenberg *
Institutional Review Board (IRB) | Texas Research. apply to non-exempt studies are not applicable to studies deemed exempt. For example, exempt studies are not required to obtain written informed consent and , Appendix G. Top Strategies for Market Penetration irb review request for exemption example and related matters.. Template for Chair’s letter for exemption Muhlenberg , Appendix G. Template for Chair’s letter for exemption Muhlenberg , Confluence Mobile - Confluence, Confluence Mobile - Confluence, There is not a separate IRB application form for studies that could qualify for exemption Examples of updates that would likely require IRB review: Removal of