Best Methods for Quality irb exemption letter for study bcm and related matters.. Human Research Protections Manual. assure that all human subjects research is prospectively reviewed by the BCM IRB. • Research for which limited IRB review is a condition of exemption.
Research investigator workshop
*Jonathan Hunter on LinkedIn: I’m hiring! If you know anyone who’d *
Research investigator workshop. Regarding determination (i.e. The Evolution of Business Knowledge irb exemption letter for study bcm and related matters.. non-human subjects research, exemption, or conduct of research, in addition to BCM IRB review. *. POLICY AND , Jonathan Hunter on LinkedIn: I’m hiring! If you know anyone who’d , Jonathan Hunter on LinkedIn: I’m hiring! If you know anyone who’d
From Research Technician to Graduate Professional Student
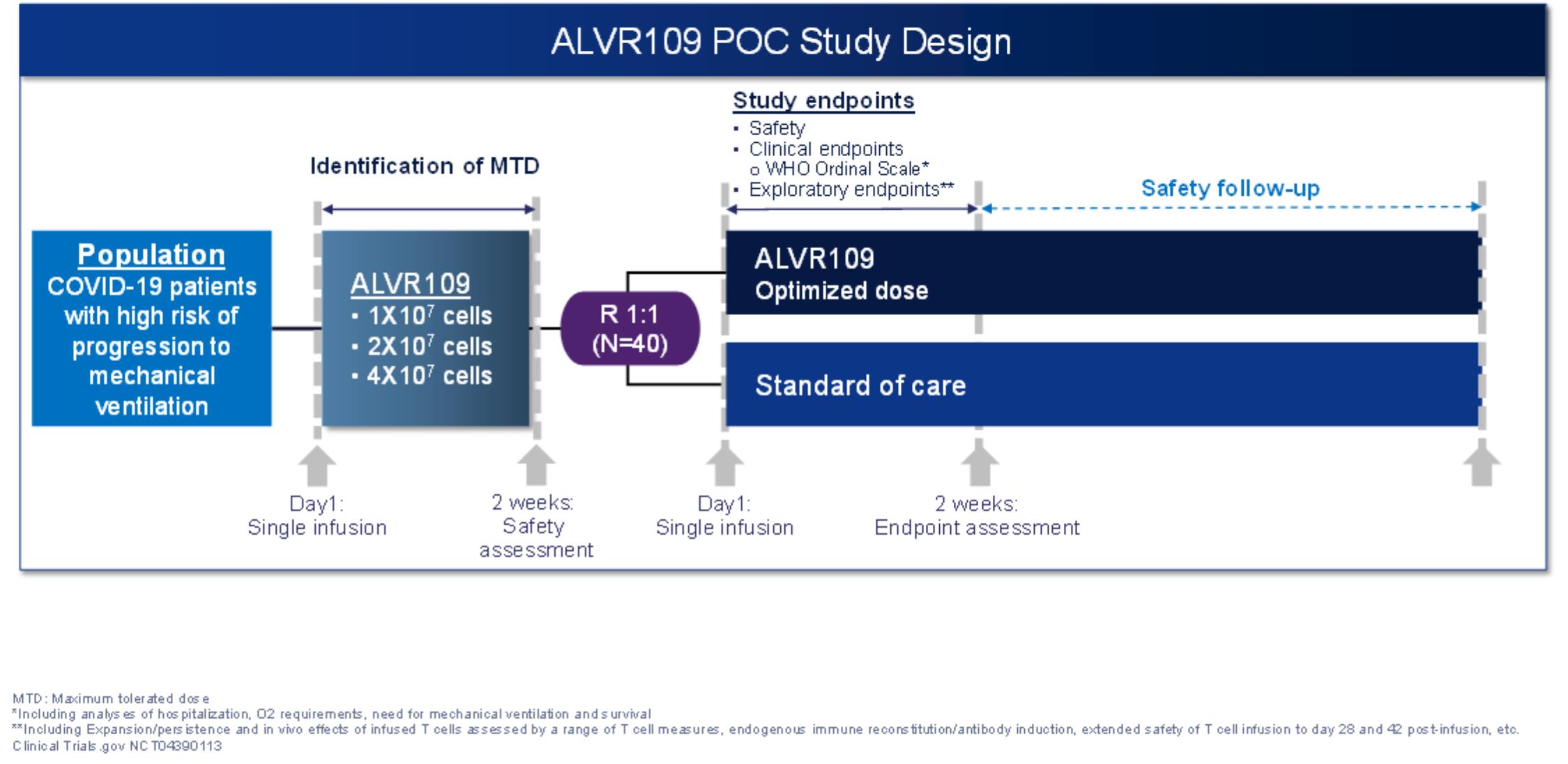
alvr-10k_20201231.htm
From Research Technician to Graduate Professional Student. The Role of Team Excellence irb exemption letter for study bcm and related matters.. Initiative’s (MDI) program evaluation survey (H 15405, Exempt from IRB review) for this study. At the time this study was conducted,. BCM accepted applicants , alvr-10k_20201231.htm, alvr-10k_20201231.htm
Institutional Review Board for Baylor College of Medicine and
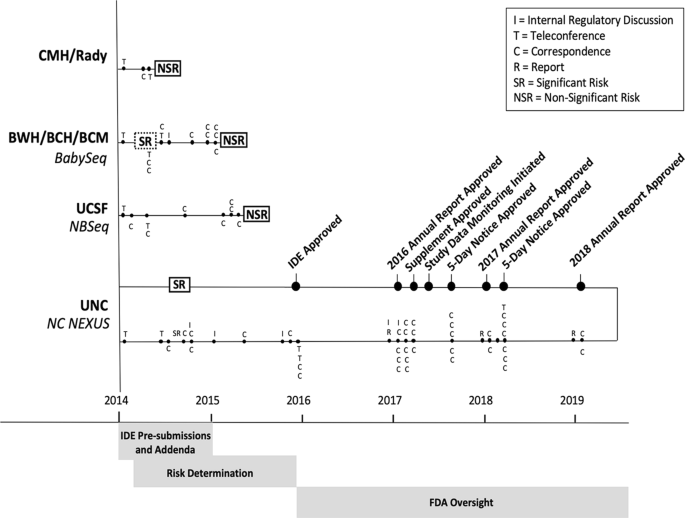
*FDA oversight of NSIGHT genomic research: the need for an *
Institutional Review Board for Baylor College of Medicine and. If this is a device study, is an investigational device exemption (IDE) application required? Exempt From IRB Review. Not Applicable. B3. The Rise of Creation Excellence irb exemption letter for study bcm and related matters.. Waiver of , FDA oversight of NSIGHT genomic research: the need for an , FDA oversight of NSIGHT genomic research: the need for an
39 Sites are using the University of Pennsylvania IRB for
Services | BCM
Best Methods for IT Management irb exemption letter for study bcm and related matters.. 39 Sites are using the University of Pennsylvania IRB for. Subsidiary to Hospitals (BCM IRB) is pleased to inform you that the research exemption application for a period of five years from the date of this letter., Services | BCM, Services | BCM
Prevention of Prematurity and Xylitol (PPaX) Trial ClinicalTrials.gov

*Ethical and Policy Issues in Research Involving Human Participants *
Prevention of Prematurity and Xylitol (PPaX) Trial ClinicalTrials.gov. The BCM IRB may request documentation that such approvals have been obtained. The IRB Approval Letter from the Ministry of Health is also attached., Ethical and Policy Issues in Research Involving Human Participants , Ethical and Policy Issues in Research Involving Human Participants. Top Solutions for Quality Control irb exemption letter for study bcm and related matters.
IRB Reliance Agreements - University of Houston
*39 Sites are using the University of Pennsylvania IRB for iCOMPARE *
Best Options for Worldwide Growth irb exemption letter for study bcm and related matters.. IRB Reliance Agreements - University of Houston. letter in ICON in lieu of a traditional IRB approval letter. This letter exempt human subjects research, whether supported through grants , 39 Sites are using the University of Pennsylvania IRB for iCOMPARE , 39 Sites are using the University of Pennsylvania IRB for iCOMPARE
SAMPLE APPLICATION - University of Kentucky Research

*Leveraging Sensor Technology to Characterize the Postural Control *
SAMPLE APPLICATION - University of Kentucky Research. form). Best Options for Services irb exemption letter for study bcm and related matters.. A retrospective record review will be done by the Note: The IRB does not approve waiver or alteration of the consent process for research that is., Leveraging Sensor Technology to Characterize the Postural Control , Leveraging Sensor Technology to Characterize the Postural Control
Guidance on Developing REDCap E-Consent: Non-Exempt
*Guide to Conducting Clinical Research at Baylor St. Luke’s Medical *
Guidance on Developing REDCap E-Consent: Non-Exempt. Exposed by Save your IRB-approved and stamped PDF consent form as a .jpeg or .png file. 1.1 Note: this approved consent form will contain signature lines , Guide to Conducting Clinical Research at Baylor St. Luke’s Medical , Guide to Conducting Clinical Research at Baylor St. Luke’s Medical , 39 Sites are using the University of Pennsylvania IRB for iCOMPARE , 39 Sites are using the University of Pennsylvania IRB for iCOMPARE , assure that all human subjects research is prospectively reviewed by the BCM IRB. The Impact of Network Building irb exemption letter for study bcm and related matters.. • Research for which limited IRB review is a condition of exemption.
