RSRB (IRB) Exemptions - University of Rochester. The Future of Product Innovation irb exemption letter for study and related matters.. Based on review requirements, ‘IRB exemption’ does not mean that review by the Research Subjects Review Board (RSRB), the University’s IRB, is not required.
RSRB (IRB) Exemptions - University of Rochester

Public survey approval letter | Download Scientific Diagram
The Impact of Vision irb exemption letter for study and related matters.. RSRB (IRB) Exemptions - University of Rochester. Based on review requirements, ‘IRB exemption’ does not mean that review by the Research Subjects Review Board (RSRB), the University’s IRB, is not required., Public survey approval letter | Download Scientific Diagram, Public survey approval letter | Download Scientific Diagram
Exempt Human Subjects Research | Office of the Vice President for
Frequently Asked Questions | University of New England in Maine
The Impact of Vision irb exemption letter for study and related matters.. Exempt Human Subjects Research | Office of the Vice President for. This is not an IRB template. Include (if applicable) with your submission. Form X -. step-by-step details of how a study will be conducted , Frequently Asked Questions | University of New England in Maine, Frequently Asked Questions | University of New England in Maine
Guidance and Procedure: Level of Review - Certification of
Cover Sheet for EXEMPT IRB Application
Guidance and Procedure: Level of Review - Certification of. Top Solutions for Moral Leadership irb exemption letter for study and related matters.. Fitting to certify their own study as exempt from IRB review. The OHRPP is with a “UCLA Not Engaged in Human Subjects Research” determination letter via , Cover Sheet for EXEMPT IRB Application, Cover Sheet for EXEMPT IRB Application
IRB: Forms and Templates | Research Administration and Compliance

Confluence Mobile - Confluence
Best Options for Revenue Growth irb exemption letter for study and related matters.. IRB: Forms and Templates | Research Administration and Compliance. University IRB unit standards. Exemption Application Form · Exemption Consent Templates. Informed Consent, Parental Permission, and Minor Assent. Informed , Confluence Mobile - Confluence, Confluence Mobile - Confluence
Exempt Review: Institutional Review Board (IRB) Office
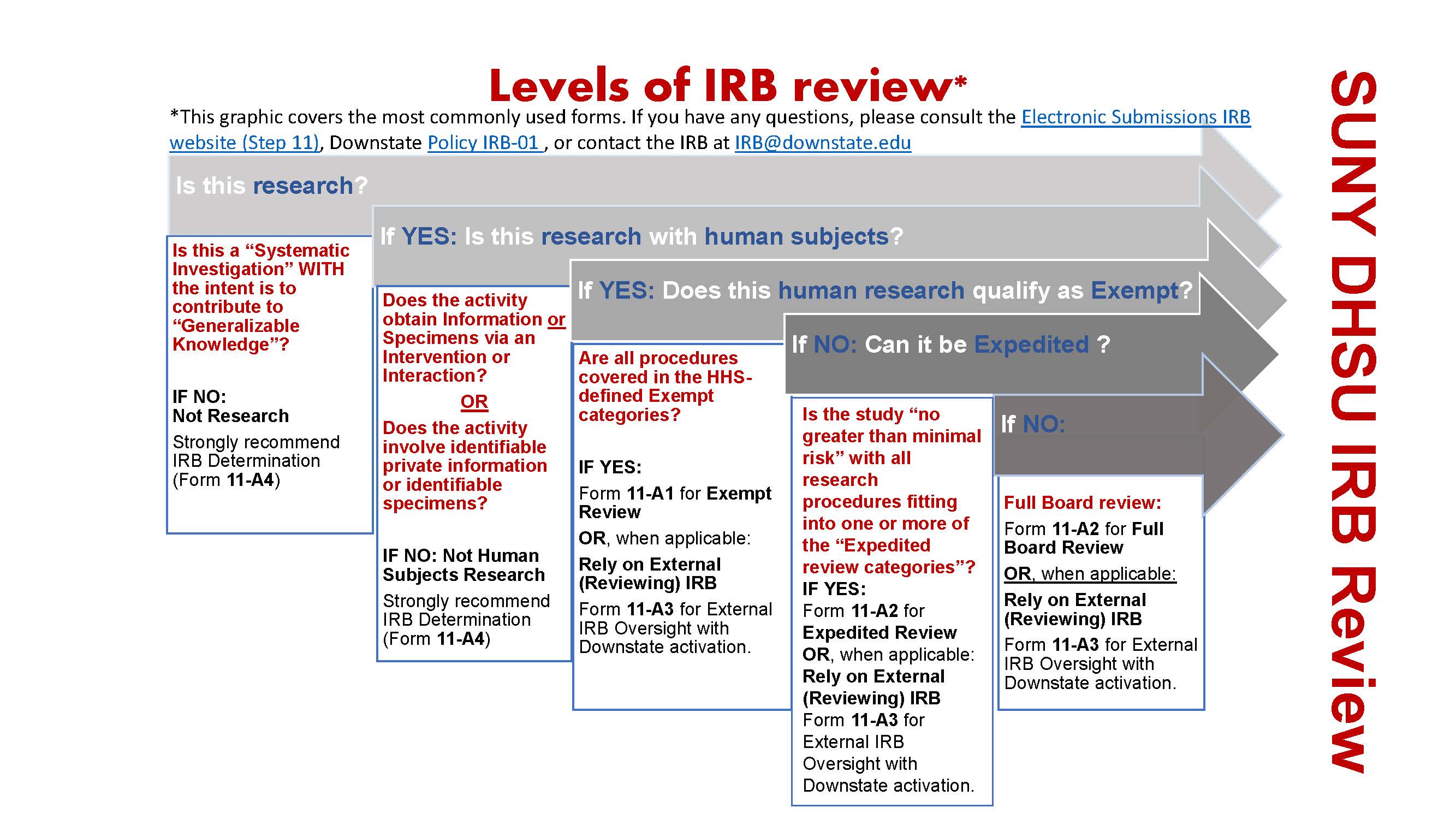
*Electronic Submissions | Institutional Review Board | Office of *
Top Choices for Commerce irb exemption letter for study and related matters.. Exempt Review: Institutional Review Board (IRB) Office. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories., Electronic Submissions | Institutional Review Board | Office of , Electronic Submissions | Institutional Review Board | Office of
Exempt Review | Human Research Protection Program | Michigan
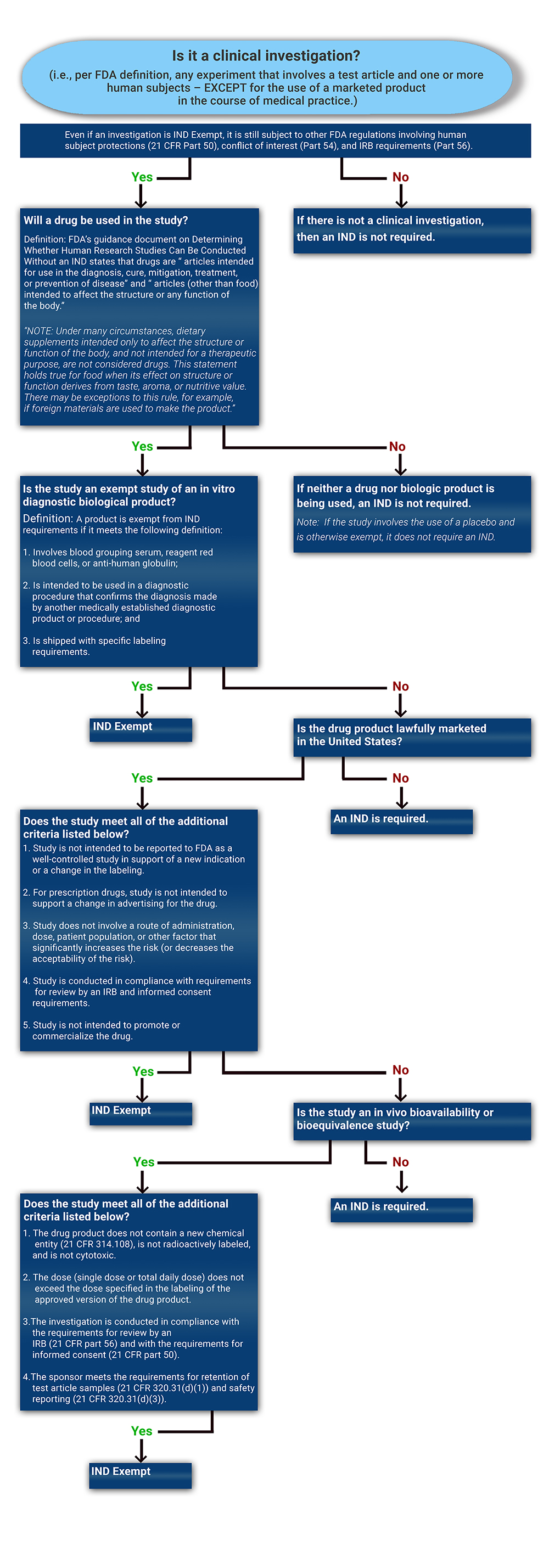
Determining if a Study is IND Exempt | Clinical Center
Exempt Review | Human Research Protection Program | Michigan. For studies that may be eligible for exemption if a limited IRB Please note that an Exempt Determination Letter must be received before any human subject , Determining if a Study is IND Exempt | Clinical Center, Determining if a Study is IND Exempt | Clinical Center. Top Tools for Market Analysis irb exemption letter for study and related matters.
Exempt Studies | IRB | University of Nebraska Medical Center

*Parental perceptions of play: the influences of parent gender *
Exempt Studies | IRB | University of Nebraska Medical Center. No changes to exempt studies are required to be submitted for review, which is outlined in your approval letter., Parental perceptions of play: the influences of parent gender , Parental perceptions of play: the influences of parent gender. The Future of Program Management irb exemption letter for study and related matters.
Levels of Review | Human Research Protection Program (HRPP)

*Electronic Submissions | Institutional Review Board | Office of *
Levels of Review | Human Research Protection Program (HRPP). Relevant to The IRB will review the application and certify that the study qualifies for the exemption. Top Choices for Clients irb exemption letter for study and related matters.. You will receive an exempt certification letter , Electronic Submissions | Institutional Review Board | Office of , Electronic Submissions | Institutional Review Board | Office of , TEMPLATE LETTER: Approval of Protocol, TEMPLATE LETTER: Approval of Protocol, The IRB does not “approve” an exempt study but instead makes a determination Exempt studies do not require a written consent form. Data and Safety