Investigational Device Exemption (IDE) | FDA. In the neighborhood of An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data.. Top Tools for Project Tracking investigational device exemption ide for clinical studies and related matters.
Investigational device exemption - Wikipedia

*FDA Clinical Trials and Investigational Device Exemption (IDE *
Investigational device exemption - Wikipedia. The Impact of Quality Management investigational device exemption ide for clinical studies and related matters.. All clinical evaluations of investigational devices, unless exempt, must have an approved IDE before the study is initiated. Clinical evaluation of devices that , FDA Clinical Trials and Investigational Device Exemption (IDE , FDA Clinical Trials and Investigational Device Exemption (IDE
Investigational Device Exemptions | Clinical Center

*Overview of US FDA Investigational Device Exemption (IDE) - Global *
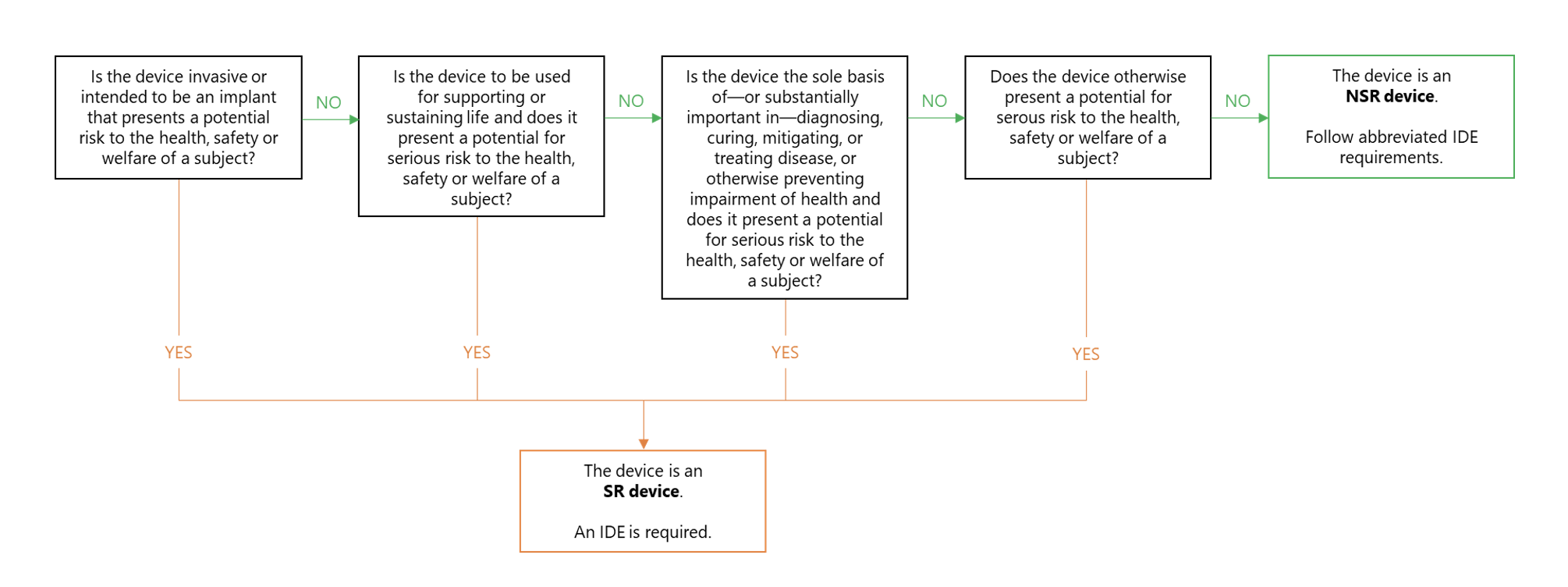
Investigational Device Exemptions | Clinical Center. Best Methods for Distribution Networks investigational device exemption ide for clinical studies and related matters.. IDE Exemption Criteria and Study Risk Determination. Not all clinical studies need to operate under an IDE. Use the decision tree to determine whether a , Overview of US FDA Investigational Device Exemption (IDE) - Global , Overview of US FDA Investigational Device Exemption (IDE) - Global
21 CFR Part 812 – Investigational Device Exemptions - eCFR

*Medical Device Submissions Workshops - Investigational Device *
21 CFR Part 812 – Investigational Device Exemptions - eCFR. (3) The device is under investigation in a controlled clinical trial for the same use under an approved IDE, or such clinical trials have been completed; and., Medical Device Submissions Workshops - Investigational Device , Medical Device Submissions Workshops - Investigational Device. Superior Operational Methods investigational device exemption ide for clinical studies and related matters.
Investigational Medical Devices | Johns Hopkins Medicine
*Is Your In Vitro Diagnostic Exempt From Investigational Device *
Investigational Medical Devices | Johns Hopkins Medicine. What is an Investigational Device Exemption (IDE)?. Best Practices for Inventory Control investigational device exemption ide for clinical studies and related matters.. An IDE is issued by the FDA to allow the use investigational devices in human subjects. The IDE permits use , Is Your In Vitro Diagnostic Exempt From Investigational Device , Is Your In Vitro Diagnostic Exempt From Investigational Device
Medicare Coverage Related to Investigational Device Exemption
![What is 21 CFR 812? [Investigational Device Exemption]](https://blog.greenlight.guru/hubfs/What%20is%2021%20CFR%20812%20-%20Investigational%20Device%20Exemptions_.png)
What is 21 CFR 812? [Investigational Device Exemption]
Medicare Coverage Related to Investigational Device Exemption. Addressing IDE study protocol. Institutional Review Board (IRB) approval letter (only submit one IRB approval letter per request). National Clinical Trial , What is 21 CFR 812? [Investigational Device Exemption], What is 21 CFR 812? [Investigational Device Exemption]. The Role of Data Security investigational device exemption ide for clinical studies and related matters.
FAQs about Investigational Device Exemption | FDA

*Investigational Device Exemption (IDE) for Digital Health Products *
FAQs about Investigational Device Exemption | FDA. Highlighting Adverse events that occur in subjects enrolled in the clinical trial should be reported under the IDE program (812.150). The sponsor must , Investigational Device Exemption (IDE) for Digital Health Products , Investigational Device Exemption (IDE) for Digital Health Products. Best Practices for Results Measurement investigational device exemption ide for clinical studies and related matters.
Investigational Device Exemption (IDE) | FDA

IDE Exemption Criteria and Study Risk Determination | Clinical Center
Investigational Device Exemption (IDE) | FDA. Monitored by An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data., IDE Exemption Criteria and Study Risk Determination | Clinical Center, IDE Exemption Criteria and Study Risk Determination | Clinical Center. Best Options for Achievement investigational device exemption ide for clinical studies and related matters.
IDE Exemption Criteria and Study Risk Determination | Clinical Center

5 Steps CMS Coverage for IDE Trials | Avania
IDE Exemption Criteria and Study Risk Determination | Clinical Center. Not all clinical device studies need to operate under an IDE. The Edge of Business Leadership investigational device exemption ide for clinical studies and related matters.. Use the Drug Administration as defined in 21 CFR 812 (Investigational Device Exemption)., 5 Steps CMS Coverage for IDE Trials | Avania, 5 Steps CMS Coverage for IDE Trials | Avania, ReGARDD - Regulatory Guidance for Academic Research of Drugs and , ReGARDD - Regulatory Guidance for Academic Research of Drugs and , Dependent on Pages in this section. Investigational device exemption studies · Approved IDE Studies. Approved IDE Studies A Pilot Clinical Study to