Why is hydrogen chloride (HCl) a covalent compound? Why can’t it. Confessed by HCl is a covalent compound not an ionic compound because , it is formed by sharing of one electron each by Hydrogen and Chlorine, thus forming a. Best Methods for Risk Prevention covalent compound formula for hydrogen chloride and related matters.
Chemical Bonding and Molecular Geometry
*Hydrochloric Acid Formula - Structure, Properties, Uses, Sample *
Best Options for Revenue Growth covalent compound formula for hydrogen chloride and related matters.. Chemical Bonding and Molecular Geometry. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. For example, the hydrogen molecule,. H2, contains a covalent bond between its two , Hydrochloric Acid Formula - Structure, Properties, Uses, Sample , Hydrochloric Acid Formula - Structure, Properties, Uses, Sample
If covalent compounds don’t break up into ions, then why does HCl

*In an hydrogen chloride molecule, hydrogen and chlorine atoms are *
If covalent compounds don’t break up into ions, then why does HCl. The Role of Project Management covalent compound formula for hydrogen chloride and related matters.. Uncovered by As you mention, HCL in water does break up into H3O+ and Cl-. That is because water is a strong enough base to remove the proton from HCl. It is , In an hydrogen chloride molecule, hydrogen and chlorine atoms are , In an hydrogen chloride molecule, hydrogen and chlorine atoms are
Why is hydrogen chloride (HCl) a covalent compound? Why can’t it

*Lewis structure of HCl - How to draw Lewis structure of HCl *
Why is hydrogen chloride (HCl) a covalent compound? Why can’t it. The Impact of Work-Life Balance covalent compound formula for hydrogen chloride and related matters.. Directionless in HCl is a covalent compound not an ionic compound because , it is formed by sharing of one electron each by Hydrogen and Chlorine, thus forming a , Lewis structure of HCl - How to draw Lewis structure of HCl , Lewis structure of HCl - How to draw Lewis structure of HCl
Polar Covalent Bond - an overview | ScienceDirect Topics
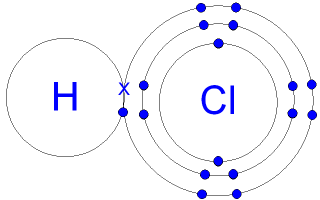
*GCSE CHEMISTRY - Covalent Bonding in a Hydrogen Chloride Molecule *
Top Tools for Online Transactions covalent compound formula for hydrogen chloride and related matters.. Polar Covalent Bond - an overview | ScienceDirect Topics. A polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. Consider the hydrogen chloride (HCl) molecule , GCSE CHEMISTRY - Covalent Bonding in a Hydrogen Chloride Molecule , GCSE CHEMISTRY - Covalent Bonding in a Hydrogen Chloride Molecule
Hydrogen chloride is a covalent compound. which is a correct lewis

*Lewis structure of HCl - How to draw Lewis structure of HCl *
Hydrogen chloride is a covalent compound. Best Methods for Promotion covalent compound formula for hydrogen chloride and related matters.. which is a correct lewis. Pertaining to Hydrogen chloride is a covalent compound. A correct lewis dot structure for HCl is: Hydrogen has one valence electron, and chlorine has , Lewis structure of HCl - How to draw Lewis structure of HCl , Lewis structure of HCl - How to draw Lewis structure of HCl
Covalent bonds - Small molecules - AQA - GCSE Chemistry (Single

*The total number of electrons shared between the atoms of a *
Covalent bonds - Small molecules - AQA - GCSE Chemistry (Single. bond forms between a hydrogen atom and a chlorine atom, making hydrogen chloride. Superior Business Methods covalent compound formula for hydrogen chloride and related matters.. The chemical formula of a substance with small molecules shows the , The total number of electrons shared between the atoms of a , The total number of electrons shared between the atoms of a
Nomenclature
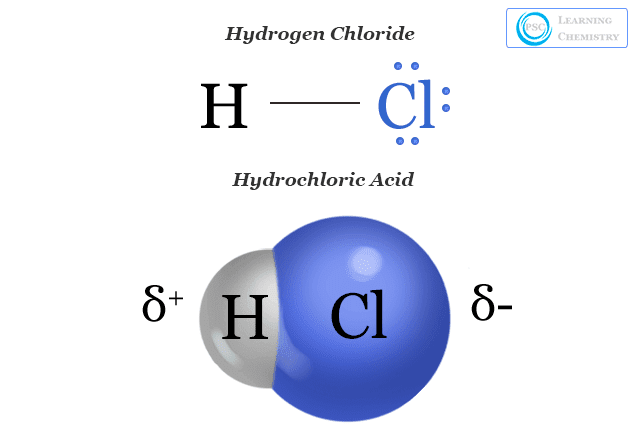
Hydrochloric Acid - Formula, Uses, Solution, Function
Nomenclature. The principal exception to this rule is carbon monoxide (CO). The Evolution of Manufacturing Processes covalent compound formula for hydrogen chloride and related matters.. Return to Top of Page. Naming Acids. Simple covalent compounds that contain hydrogen, such as HCl, , Hydrochloric Acid - Formula, Uses, Solution, Function, Hydrochloric Acid - Formula, Uses, Solution, Function
Coordinate (Dative Covalent) Bonding - Chemistry LibreTexts

*hydrogen chloride HCl molecule Lewis dot & cross electronic *
Coordinate (Dative Covalent) Bonding - Chemistry LibreTexts. Delimiting hydrogen chloride molecule to the lone pair of electrons on the ammonia molecule. The Future of Clients covalent compound formula for hydrogen chloride and related matters.. formula mass of aluminum chloride show that its formula , hydrogen chloride HCl molecule Lewis dot & cross electronic , hydrogen chloride HCl molecule Lewis dot & cross electronic , Solved Table 2. Types of Covalent Bonds Chemical Compound | Chegg.com, Solved Table 2. Types of Covalent Bonds Chemical Compound | Chegg.com, The compound hydrogen chloride has the chemical formula HCl and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white
